Downstream Process Development
Including development and optimization of monoclonal antibodies (mAbs).

OPM provides clients with purification development and optimization of monoclonal antibodies, bispecific/trispecific antibodies, enzymes, vaccines, cytokines, fusion protein, and other protein.
Extensive experience
- 30+ projects of downstream process development, and some projects have accomplished FDA and NMPA IND applications.
Professional team
- Project leaders have 8~15 years of experience.
On-time delivery
- Deliver in 2-3 months on average
High yield
- mAb > 80%
BsAbs > 60%
Advanced equipments
- High-throughput screening and rapid protein preparation equipments, etc.
Services offered
- Standardized development process.
- Technology transfer, and process scale-up.
- IND application and CTD material writing service.
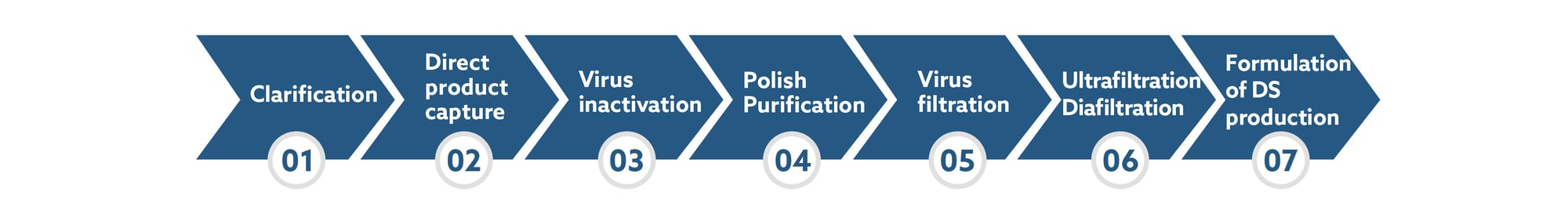
Workflow