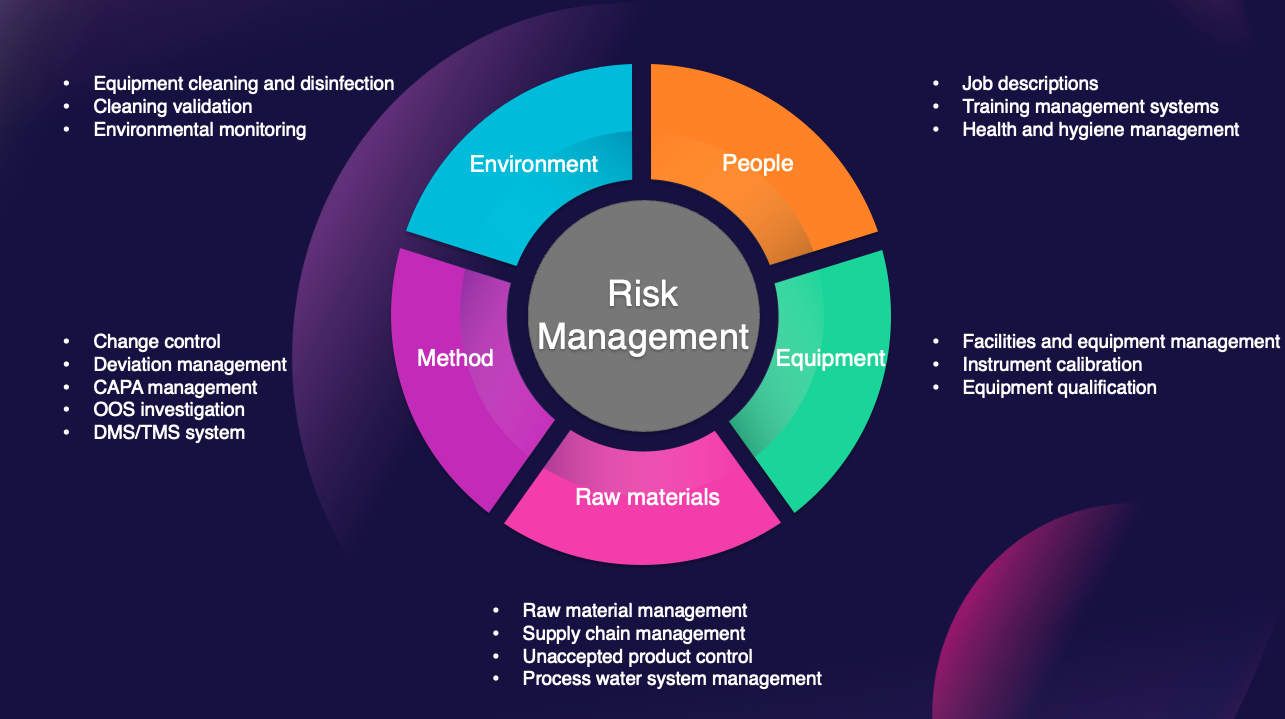
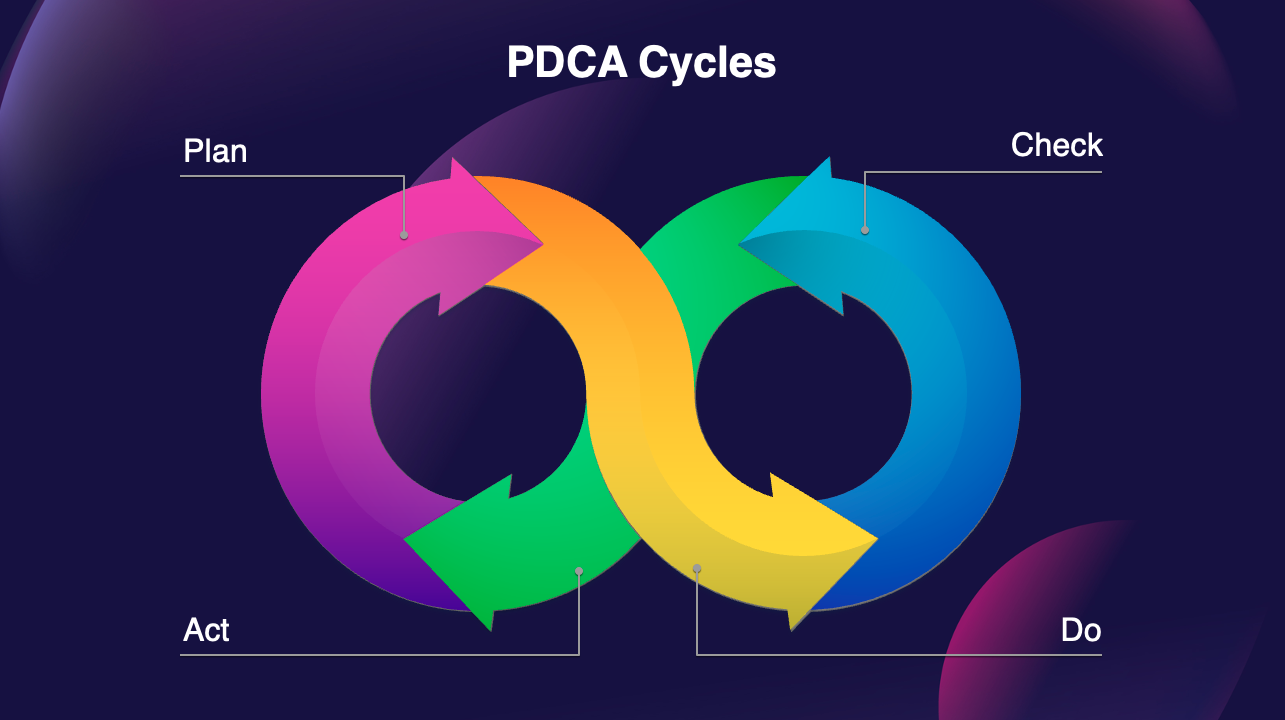
Quality management system
Designed to ensure excellence throughout the entire pharmaceutical life cycle,
following the GMP requirements for the United States, Europe and Asia.
At OPM, we have implemented a robust quality management system, certified to ISO 9001 and ISO 13485 standards, that is fully compliant with Good Manufacturing Practices (GMP)