Stable Cell Line Development
Targeting a wide range of macro molecular drugs (mono-, bi-, tri-antibodies, fusion proteins, enzymes, vaccines, etc.)

Efficient stable cell line development is a key step to obtain high-quality protein, as well as for the CMC development. OPM provides comprehensive and professional stable cell line development service to support IND application.
Host cells
- Wild type: ATCC CHO-Kl, ECACC CHO-Kl, CHOZN CHOK1, CHO-S, etc.
- Knockout type: CHOZN GS, DG44, etc.
- GS screening system, DHFR screening system and novel screening system.
- Host cell systems supplied by clients.
Biologics development
- Completed 100+ cell line development projects for therapeutic protein expression.
- Including monoclonal antibodies, bispecific antibodies, tri-specific antibodies, fusion proteins, vaccines, enzymes, cytokines and novel structure proteins.
Fast, high-titer and high-quality development
- Protein samples delivered within 5 weeks with desired CQAs such as protein quality and titer.
- Delivery of monoclonal cells in less than 8 weeks.
- mAb titer: 3-10 g/ L, bispecific antibody titer: 2-10 g/ L, vaccine titer: 1-10 g/ L.
Professional and compliant development process
- Provide host cells of documented origins.
Data management: Integrity, authenticity and traceability. - Provide clonality report to speed up the application process.
- 20+ IND applications accomplished in China, US and Australia.
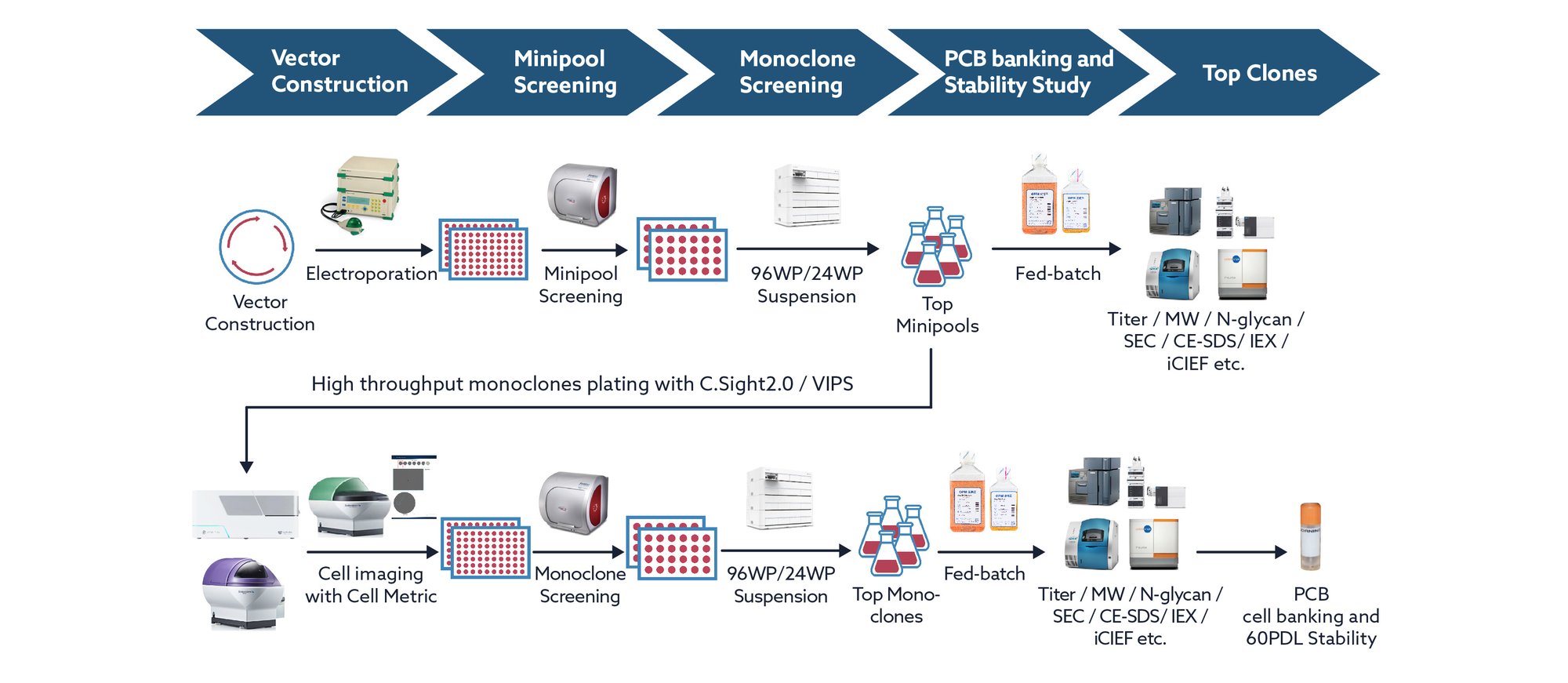
Workflow