SERVICES
Custom services
Expertly designed with your cell line in mind

Tailored cell culture media solutions for your specific needs
OPM offers fully customized cell culture media development services, designed to meet the specific needs of various cell lines, such as CHO-K1, CHO-S, CHO-DG44, and CHOZN. Our custom media solutions are designed to boost protein titer, enhance protein quality (such as glycoforms, charge distribution, and fragmentation), and improve overall process efficiency.
-
10+ years of expertise in cell culture media development
-
Proven scalability with verification in 3L-10L bioreactors
-
Successful track record of custom media development with over 60 clients globally
-
Seamless tech transfer to custom cGMP manufacturing if required
-
High-throughput screening platform, delivering results within 3-6 months
-
Expert input for downstream process development
-
Comprehensive, reliable data management through our online process information system
-
Superior batch consistency with an RSD of < 5%
Examples of media development programs
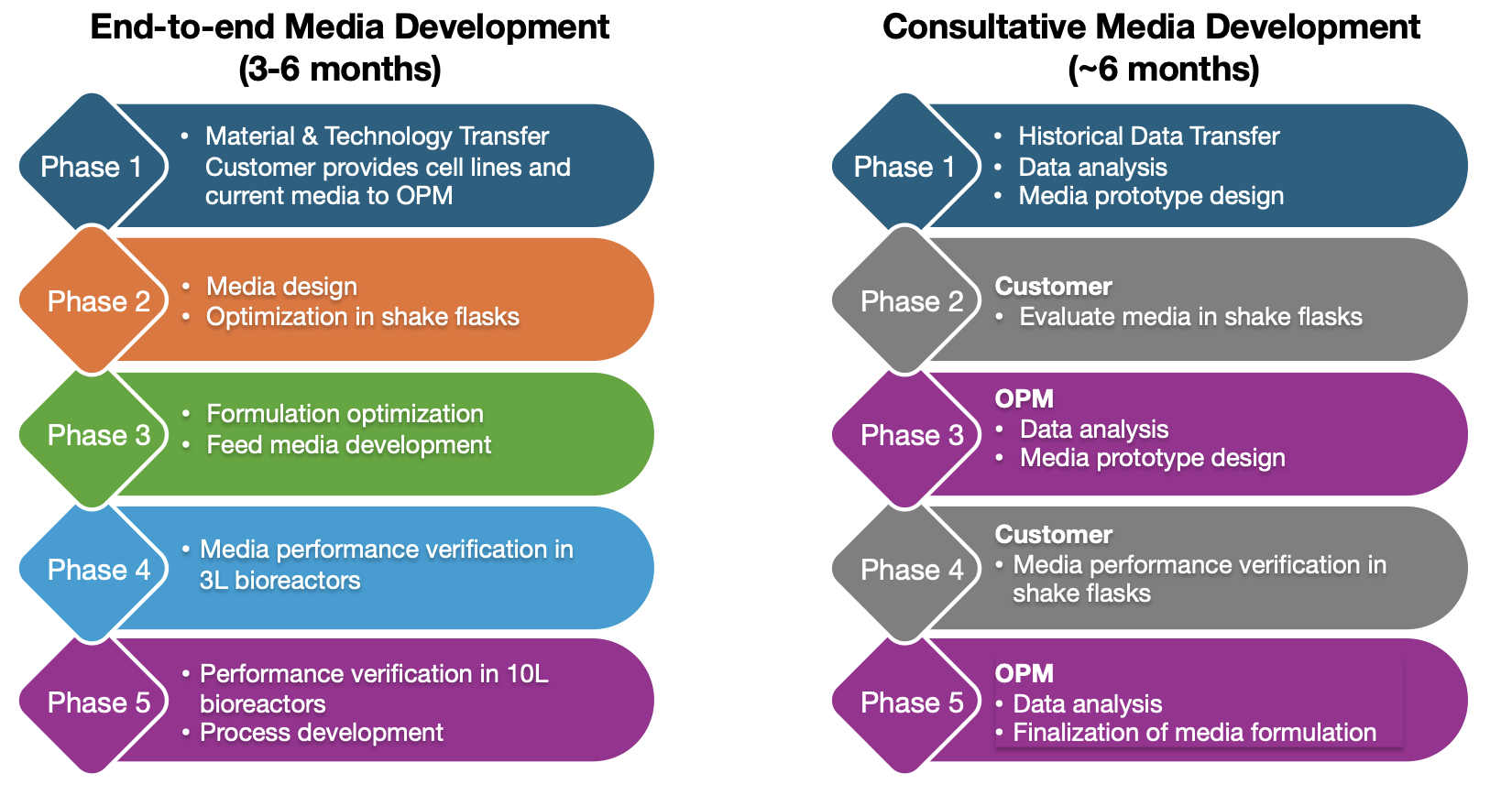
Leverage OPM’s expertise to optimize your cell culture media and achieve higher performance, better protein quality, and accelerate development timelines.
Custom cGMP manufacturing solution
Leverage our extensive experience in media development and large-scale production with our Custom cGMP Manufacturing service. Partner with us to manufacture your custom media, so you can focus on your next regulatory milestone. With industry lead times around 8 weeks, we understand speed is important for our customers.
-
Large-scale cGMP manufacturing: dry powder up to 2,000 kg and liquid media up to 2,000 L batch sizes
-
Certified manufacturing facilities: ISO9001:2015 certified and GMP compliant
-
Superior batch consistency: achieved RSD of < 5%
-
Cell culture media solutions: if your media isn’t meeting your performance expectations and requires further optimization
