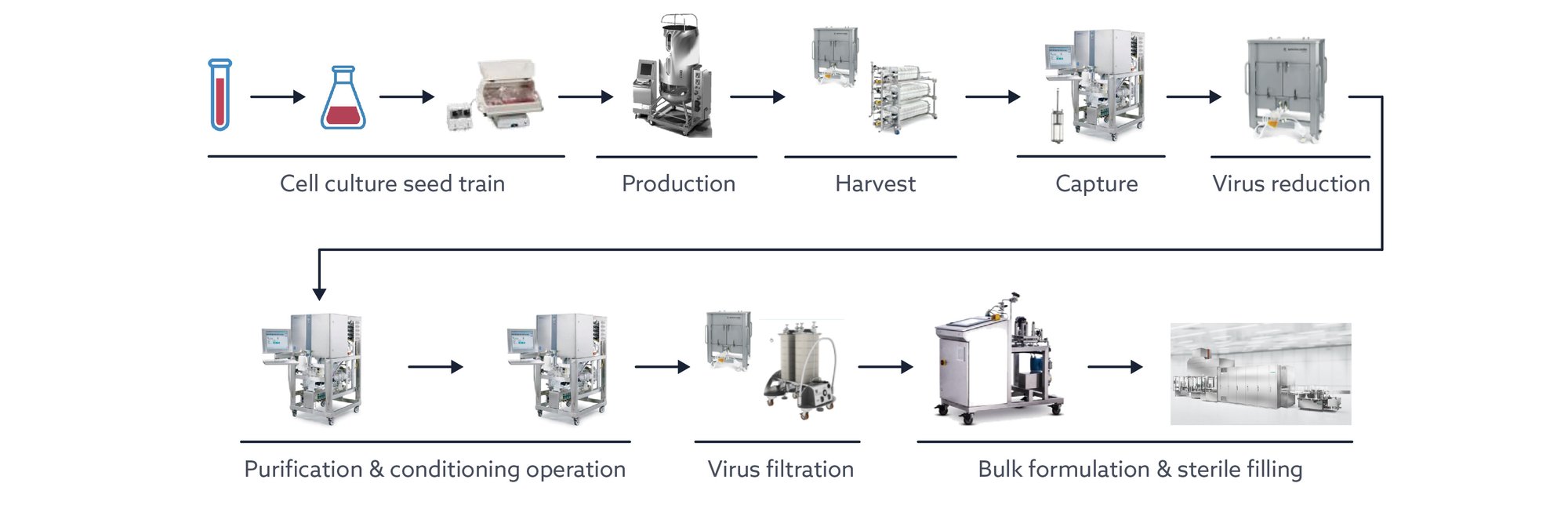
Manufacturing
GMP manufacturing including preclinical, clinical phases I/II/III, and commercial DS or DP

Production advantages
High performance media
- High-performance media can reduce manufacturing cost.
Advanced equipments
- Use mainstream international single-use bioreactors, liquid preparation and storage equipment to effectively reduce the risk of cross-contamination.
Rapid technology transfer
- Technology transfered in three months.
Drug substance (DS) manufacturing
- Two 2000 L production lines, and two 200 L / 500 L Manufacturing lines.
Drug Product (DP) filling
- A liquid fill-finish production line: Syntegon (formerly Bosch) filling line, 300 bottles/minute, specifications 2 mL / 6 mL / 10mL.
GMP Manufacturing
- Pre-clinical
- Clinical phases I, II, III
- Commercial DS or DP
Workflow