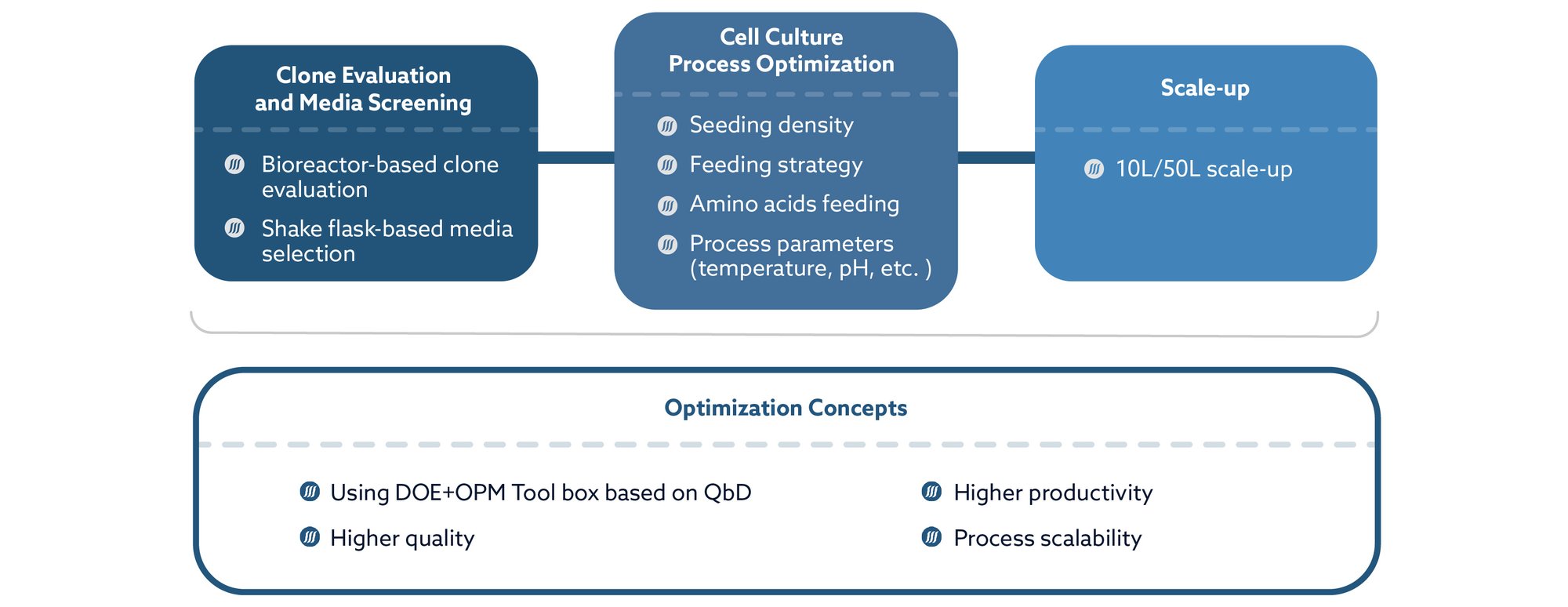
Upstream Process Development
Including development, optimization, and characterization

OPM is proficient at productivity boosting and quality fine tunning for mAbs, BsAb, fusion protein and enzyme. We also provide technology transfer, process scale-up, late-stage process development and process characterization. IND application and CTD material writing service.
Extensive experience
- 80+ projects of upstream process development, involving monoclonal antibodies, multi antibodies, fusion proteins, enzymes, etc.
- The maximum expression level has increased by over 900%.
- 10+ projects have accomplished FDA and NMPA IND application.
Professional team
- Upstream team members have 8-15 years of experience in developing biopharmaceutical projects.
- Upstream process development can be completed within 1.5 months.
All stage process development
- Early and late stage process development and characterization
Advanced equipments
- More than 30 sets of 3 L to 50 L bioreactors, more than 10 shakers, equipped with multi-brand supportive equipments.
Multiple targets
- Innovator or Biosimilar.
- QA improvement/ modification.
- mAb/BsAb/recombinant protein.
Different modes
- Fed-batch
- Intensified fed-batch
- Perfusion
- High throughput
Workflow